Properties of Sodium carboxymethylcellulose
1. Structure
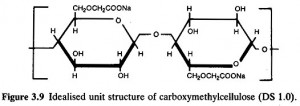
Sodium carboxymethylcellulose is typical ionic-type cellulose ether and the frequently used product is its sodium salt, as well as ammonium and aluminum salts. Sometimes, Sodium carboxymethylcellulose acids can be produced. When degree of substitution (that is, the average value of hydroxyl groups reacted with the substitution of each anhydrous glucose monomer) is 1, its molecular formula is [C6H7O2 (OH) 2OCH2COONa] n. With drying at the temperature of 105℃ and constant weight, the content of sodium is 6.98-8.5%.
2. Appearance and Solubility
The pure Sodium Carboxymethyl Cellulose is white or milk white fibrous powder or particles, odorless and tasteless. It is insoluble in organic solvents such as methanol, alcohol, diethyl ether, acetone, chloroform and benzene but soluble in water. Degree of substitution is an important factor influencing water solubility and the viscosity of Sodium carboxymethylcellulose also has a great effect on the water solubility.
In general when the viscosity is within 25-50Pa•s and the degree of substitution is about 0.3, it shows alkaline solubility and while the degree of substitution is over 0.4, it shows water solubility. With the rise of DS, the transparency of solution improves accordingly. In addition, the replacement homogeneity also has an great effect on the solubility.
3. Hygroscopicity
Sodium carboxymethylcellulose equilibrium water content will increase with the rise of air humidity but decrease with the rise of temperature. At room temperature and average humidity of 80-85%, the equilibrium water content is more than 26% but moisture content in the products is lower than 10%, lower than the former. As far as its shape is concerned, even if the water content is about 15%, there seems no difference in appearance.
However, when the moisture content reaches above 20%, inter-particle mutual adhesion can be perceived and the higher the viscosity is, the more evident it will become. For these polarized high-molecular compounds like Sodium carboxymethylcellulose, the hygroscopic degree is not only affected by the relative humidity but also by the number of polarity. The higher the degree os substitution is, that is, the larger the number of polarity, the stronger the hygroscopicity will be. Moreover, crystallinity also affects it and the higher the crystallinity is, the smaller the hygroscopic will be.
4. Compatibility
Sodium carboxymethylcellulose has good compatibility with other kinds of water-soluble glues, softeners and resin. For example, it is compatible with animal glues, dimethoxy dimethylurea gel, Arabic gum, pectin, tragacanth gum, ethylene glycol, sorbitol, glycerol, invert sugar, soluble starch and sodium alginate. It is also compatible with casein, the compound of melamine- formaldehyde resin and ethylene glycol, urea formaldehyde ethylene glycol resin, methyl cellulose, polyvinyl alcohol (PVA), phosphate nitrilotriacetic acid, and sodium silicate but the degree is slightly poorer. 1% Sodium carboxymethylcellulose solution is compatible with most inorganic salts.
5. Dissociation Constant
In the giant polymer matrix of Sodium carboxymethylcellulose, there are plenty of electrolyzing groups (carboxymethyl groups). The acidity is similar to that of acetic acid and the dissociation constant is 5×10-5. The dissociation strength has an considerable effect on the electrical properties of Sodium carboxymethylcellulose.
6. Biochemical Properties
Although Sodium carboxymethylcellulose solution is difficult to get rotten than natural gums, under certain conditions, some microbes enable it to get rotten, especially with cellulose and taka-amylase reactions, leading to the decrease of solution viscosity. The higher the DS of Sodium carboxymethylcellulose is, the less it will be affected by enzymes and this is because the side chain linked with glucose residues prevents enzymolysis.
Since the enzyme action leads to the breakage of Sodium Carboxymethyl Cellulose main chain and generates reducing sugar, in this way the degree of polymerization will decrease and the solution viscosity will accordingly decrease. The digestive enzymes within human body can have no decomposition on Sodium carboxymethylcellulose and Sodium carboxymethylcellulose has no decomposition in acid or alkaline digestive juice.
READ MORE:Properties of HPMC (Hydroxypropyl MethylCellulose)