Influencing Factors of sodium carboxymethyl cellulose (CMC) on Stabilization of Acidified Milk Drinks
Studies found that the addition amount of sodium carboxymethyl cellulose(CMC) and its molecular parameters (such as molecular weight, degree of substitution), processing techniques (such as homogenization) have greater effects on the stability of acidified milk drinks.
3.1 Effects of the Mass Fraction of CMC on Stability of Acidified Milk Drinks
It is found by measuring the settlement quantity of different acidified milk systems and the absorption capacity of CMC, combined with the change trend of the casein micelle size and zeta potential with the decrease of pH value after adding different mass fractions of CMC that with the increase of mass fraction of CMC, the system becomes even more stable. Specifically, when acidified milk drinks do not contain CMC, aggregation occurs in casein; the settlement quantity becomes higher; and the system is unstable. When the mass fraction of CMC is 0.05%, the settlement quantity is instead higher. This is because unsaturated absorption occurs on the surface of casein. One molecular chain of CMC can be connected to two or more casein particles, and the system is more susceptible to aggregation and sedimentation due to bridging flocculation. However, when the mass fraction of CMC reaches 0.3%, with the increase in the mass fraction, the settlement quantity gets reduced and has not changed much. There exists unadsorbed CMC in the system, and this part of CMC plays a role in increasing strength, while the adsorbed CMC then plays a dual role electrostatic repulsion and steric hindrance, eventually leading to stability of system. In addition, it is also found through direct observation of acidified milk system containing high mass fraction of CMC by CLSM that the dispersion of particles in the system is more uniform.
3.2 Effects of the Molecular Weight of CMC on Stability of Acidified Milk Drinks
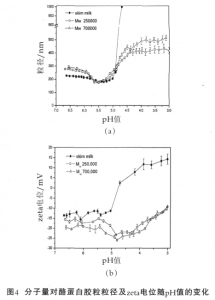
Under the condition of the same degree of substitution, at low pH value, the particle size of casein micelle in the system adding CMC of high molecular weight is larger than that adding CMC of low molecular weight, and the zeta potential is more negative (Figure 4). Since the long-chain CMC chains can form a larger “loop”, CMC of high molecular weight can be adsorbed on the surface of casein micelle, a thicker adsorption layer may be formed; the steric hindrance provided is greater; and protein micelles are more stable. Increasing the molecular weight of CMC has the same effect as increasing the mass fraction of CMC. The higher the molecular weight is, the better the stabilizing effects will be.
3.3 Effects of the Degree of Substitution of CMC on Stability of Acidified Milk Drinks
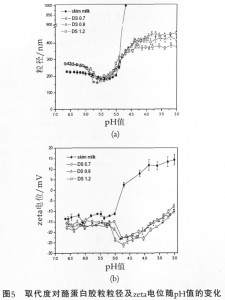
Under the condition of the same molecular weight, the higher the degree of substitution of CMC is, at low pH value, the smaller the balanced particle size of casein micelle will be and the more negative the zeta potential will be (Figure 5), so the larger the degree of substitution is, the better the stabilizing effects will be. The higher the degree of substitution of CMC is, the smaller the particle size of casein micelle in the milk system will be at low pH value, for two reasons. First, CMC of the same molecular weight and high degree of substitution has more negative charges (high charge density), making the electrostatic interaction between CMC and casein stronger, and the adsorption layer closer. Second, due to high degree of substitution, the zeta potential of casein micelles in the adsorption layer with CMC is higher, having a certain repulsive effect on the CMC to continue adsorption, and making the amount of adsorption reduced. The common result of both is to reduce the particle diameter value of casein. Studies also found that relatively speaking, the change in the degree of substitution has less effect on the stability of system, while the change in the molecular weight has greater effect on the stability of system.
3.4 Effects of Homogenization on Stability of Acidified Milk Drinks
Homogenization process is an important link affecting the stability acidified milk drinks. Sedlmeyer et al thought that in the range of 20-80 MPa for the homogenization pressure, the particle size of casein micelle will decrease with the increase of homogenization pressure, and the system will be more stable. We also found that in the range of 5-30 MPa for the homogenization pressure, the size of protein particles will also decrease with the increase of homogenization pressure, but the stability of system does not get improved due to the increase of homogenization pressure. This may be due to high pressure homogenization leading to breakage of CMC molecular chains, making its molecular weight reduced and leading to the decrease in the stiffness of system. Therefore, the homogenization pressure is not the higher the better.
There are many other factors affecting the stability, such as the molecular conformation of CMC, the homogeneity of degree of substitution, the heat treatment temperature especially the sterilization temperature and heating time, sweetener concentration, ionic strength, source of milk, and processing techniques. In addition, it should be pointed out that the effects of all the above factors on the stability of milk drinks of fermented type have different degrees of differences compared with acid adjustment type.